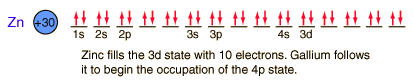
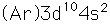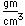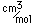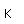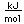Zinc
Zinc is a bluish-white, moderately hard metal. It is brittle at room temperature and at temperatures above 150°C, being workable only in the range between 100°C and 150°C. It is an active metal and will displace hydrogen even from dilute acids.
The principal use of zinc is for the galvanizing of iron sheets or wires. In moist air zinc oxidizes and becomes coated with a tough film of zinc carbonate which protects it from further corrosion. Galvanizing is done by dipping the iron into molten zinc. Zinc can also be electroplated onto iron pieces, giving a smoother galvanized surface. Sherardized iron is formed by coating the iron with and iron-zinc alloy and then baking at 800°C. The zinc coating on iron offers protection even if the zinc coating is broken by means of cathodic protection. The zinc has a more negative reduction potential (-.76V) than iron (-.04V) and therefore acts as an anode and oxidizes in preference to the iron.
Zinc is used in making alloys such as brass (the alloy with copper). Zinc is used as the outside electrode in dry cell batteries It was used in early voltaic cells such as the Daniell cell.
Zinc sulfate is used as a disinfectant and as a white pigment in paints.
Zinc oxide (ZnO) is also used as pigment called zinc white, and is used in antiseptics (zinc oxide ointment for burns). Zinc oxide is used in making rubber to improve the mechanical characteristics of the product. It is used as a constituent of the photoconductive surfaces in photocopiers. Zinc is found with manganese and iron in the oxide mineral Franklinite. Accompanying Franklinite may be the oxide mineral of zinc with calcium Hardystonite. One of the major zinc ores is the red oxide with manganese and/or iron named zincite. An oxide of zinc with manganese is Hetaerolite.
The most common zinc ore is sphalerite, ZnS. Zinc metal is produced by first roasting the ore in air to form its oxide
2ZnS(s) + 3O2(g) -> 2ZnO(s) + CO(g).
The zinc oxide is then reduced by heating it with coke (carbon) which produces the metal vapor. The vapor is condensed to the liquid form and cooled to form the solid metal
ZnO(s) + C(s) -> Zn(g) + CO(g).
The fact that the temperature required for reduction is above the boiling point for zinc (907°C) delayed the discovery of zinc. Similar reductions were used to prepare copper, lead and iron even in ancient Rome. Zinc was probably also produced, but was lost to vaporization. Brass was formed several centuries before the discovery of zinc as a pure metal (brass is a mixture of copper and zinc).
In addition to the sphalerite, zinc joins with iron to form the sulfide wurtzite, (Zn,Fe)S. Another naturally occuring ore of zinc is a carbonate mineral called Smithsonite. A striking green color is exhibited by the mineral bayldonite which combines copper, zinc and lead with an arsenate group. Another green zinc mineral is the zinc arsenate adamite, Zn2AsO4(OH). There is a dimorph of adamite called paradamite. The zinc arsenate legrandite has the same empirical formula as adamite exept for an added water of hydration and has a dramatic orange color. The zinc arsenate minerals reinerite, Zn3(AsO3)2, and kottigite lack the dramatic color.
Zinc forms the silicate willemite, Zn2SiO4 which can also produce green crystals. Zinc also forms the silicate hemimorphite. Zinc with manganese forms the silicate Hodgkinsonite. Zinc with magnesium, manganese and arsenic forms the silicate Mcgovernite. Zinc with aluminum forms the oxide mineral gahnite. Zinc is found with manganese and iron in the oxide mineral chalcophanite. An oxide with lead and vanadium is descloizite. An oxide of zinc with copper, vanadium and lead is mottramite
Zinc phosphate, Zn2PO4(OH), forms the mineral tarbuttite. Copper appears with zinc in the phosphate mineral veszelyite. Zinc appears with manganese and iron in the phosphate mineral phosphophyllite. Calcium appears with zinc in the phosphate mineral scholzite.
|