Carbon-zinc Batteries
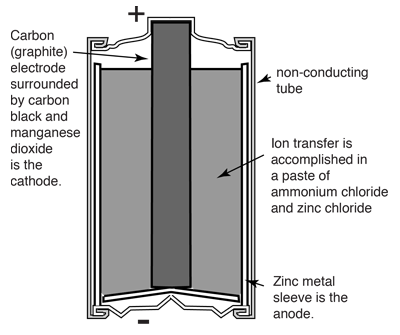
| The venerable carbon-zinc cell or Lechlanche' cell was invented in 1866 and was the most common small battery throughout most of the 20th century until largely supplanted by alkaline cells. The oxidation at the zinc electrode (the anode) is straightforward and similar to that in other cells like the Daniell cell. The other reactions involve the MnO2 which is contained near the carbon center rod and the NH4Cl and ZnCl2 which make up the bulk of the paste between the cathode and anode. |
The chemical reactions in this cell may be approximated by
Zn(s) -> Zn2+(aq) + 2e- |
|
2NH4+(aq) + 2MnO2(s) + 2e- -> Mn2O3(s) + H2O(l) + 2NH3(aq) |
|
Some of the complexity of this reaction comes from the fact that the reduction of the ammonium ion produces two gaseous products
2NH4+(aq) + 2e- -> 2NH3(g) + H2(g)
which must be absorbed to prevent the buildup of gas pressure. That is accomplished with two further reactions in the paste electrolyte. Zinc chloride reacts with ammonia to form solid zinc ammonium chloride and manganese dioxide reacts with hydrogen to form solid dimanganese trioxide plus water (Hewitt).
ZnCl2(aq) + 2NH3(g) -> Zn(NH3)2Cl2(s)
2MnO2(s) + H2(g) -> Mn2O3(s) + H2O(l)
The voltage of this cell is initially about 1.5 volts, but decreases as energy is taken from the cell. It also has a short shelf life and deteriorates rapidly in cold weather. Oxidation of the zinc wall eventually causes the contents to leak out, so such batteries should not be left in electric equipment for long periods. While these batteries have a long history of usefulness, they are declining in application since some of their problems are overcome in alkaline batteries.
| Batteries |
DC Circuits
References
Floyd
Electric Circuit Fundamentals, App. B
Ebbing
Ch 19
Hewitt
Phys. Sci. Ch 20
| HyperPhysics***** Electricity and Magnetism | R Nave |