Electronic Spectra of Molecules
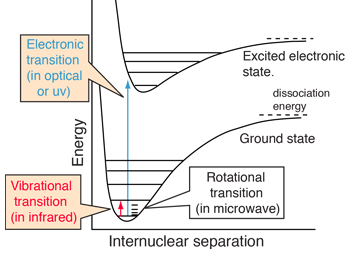 | Molecules exhibit electronic spectra from transitions between electron energy levels. These spectra are more complex than those of atomic spectra which involve transitions between electron energy levels which typically produce sharp line spectra. The energies associated with molecular electronic spectra (typically in the optical or uv region) are typically much larger than those associated with vibrational spectra (typically in the infrared) and rotational spectra (typically in the microwave region). This contributes to the complexity of the electronic spectra since the transitions from a multitude of vibrational and rotational levels produce many spectral lines, a "band" of frequencies. |
Electronic transitions are essentially instantaneous, so there is no time for appreciable motion of the nuclei. So the transitions appear as vertical lines with no change in internuclear distance. This is referred to as the Franck-Condon principle. The spectra are strongly affected by the probability that an electron is at a location to contribute to such a "vertical" transition. That probability is the wavefunction squared, and at least the lowest vibrational states are approximated by the quantum harmonic oscillator. | 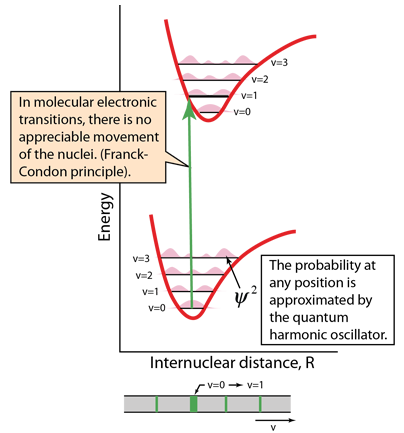 |
The energy level diagrams contain much information about the molecule. The v=0 ground ground vibrational state has the highest probability at the center of the potential well, but the higher vibrational states have the highest probabilty at maximum displacement from that center. The transition above demonstrates one result of those facts: the center of the ground state is at the same internuclear distance as the v=1 state of the first electronic excited state. So that transition is highly probably and produces a strong spectral line. Transitions involving other vibrational states are observed, but significantly weaker.
The illustration of the v=0 to v=1 transition above shows it as being broader, which is partly due to its greater intensity, but also because there can be many molecular rotational states associated with the vibrational states, giving a band of many transitions close to the same frequency. The higher inherent intensity makes more of those transitions visible and broadens the line.
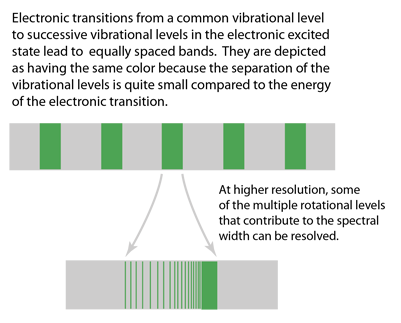 | This is a depiction of multiple electronic transitions from a common vibrational state in the ground electronic state to successive vibrational levels in an excited electronic state. There are no limiting selection rules, so transitions between many pairs of levels can occur. The lines are broadened because of the multiple rotation states associated with the vibrational levels. |
The representation above was patterned after Taylor, Zafiritos and Dubson's treatment. The section of an actual photographic record of such a spectrum at right is from Blatt and attributed to J. A. Marquisce. It is a portion of the emission spectrum of N2. | 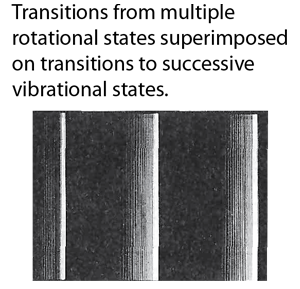 |
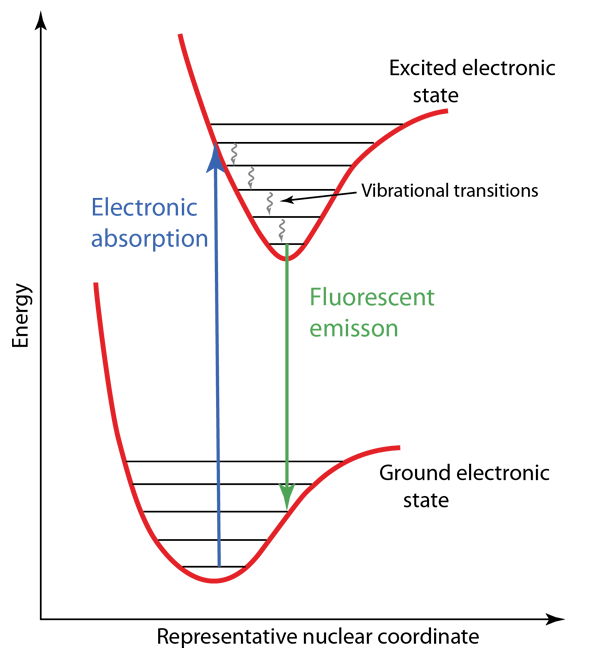
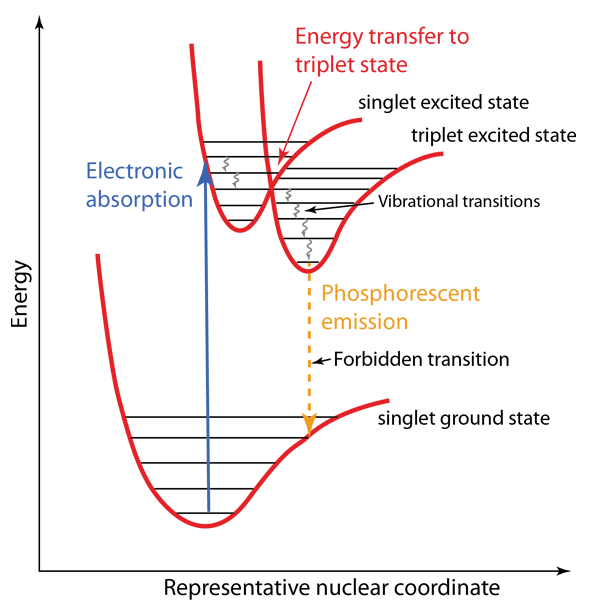
The Franck-Condon principle is an important part of the understanding of molecular electronic spectra. The relevant internuclear separations in an electronic excited state may be essentially the same as in the ground state, but if they are different, it has major effects on the nature of the electronic spectrum. Such differences may lead to the phenomenon of fluorescence in some molecules, and phosphorescence in others.
| Chemical bonds |
Molecular spectra concepts
Beiser, Perspectives of Modern Physics
Sec 14.8
Taylor, et al
Ch 12
Blatt
Ch 10
| HyperPhysics***** Quantum Physics | R Nave |