Molecular Spectra
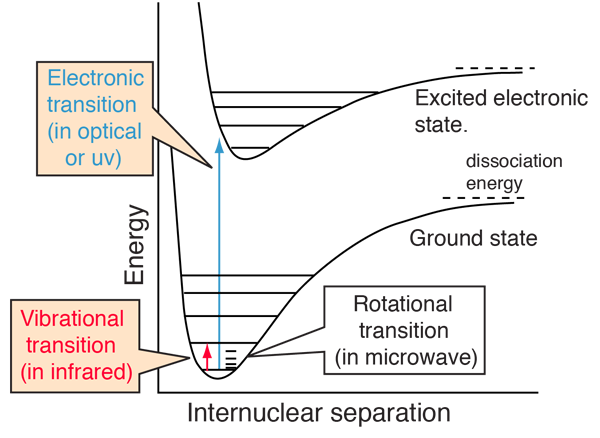 The most commonly observed molecular spectra involve electronic, vibrational, or rotational transitions. For a diatomic molecule, the electronic states can be represented by plots of potential energy as a function of internuclear distance. Electronic transitions are vertical or almost vertical lines on such a plot since the electronic transition occurs so rapidly that the internuclear distance can't change much in the process. Vibrational transitions occur between different vibrational levels of the same electronic state. Rotational transitions occur mostly between rotational levels of the same vibrational state, although there are many examples of combination vibration-rotation transitions for light molecules.
|
Index
Molecular spectra concepts |