Vibrational Spectra of Diatomic Molecules
The lowest vibrational transitions of diatomic molecules approximate the quantum harmonic oscillator and can be used to imply the bond force constants for small oscillations.
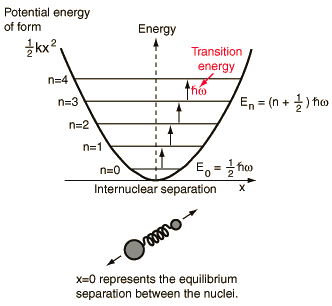 |
The following is a sampling of transition frequencies from the n=0 to n=1 vibrational level for diatomic molecules and the calculated force constants.
|
These bond force constants were calculated from the vibrational frequency in the same way the force constant for HCl was calculated.
| Vibration-rotation spectrum of HCl | HBr |
Schrodinger equation concepts
Molecular spectra concepts
References
Thornton & Rex
Sec 11-1
Barrow
| HyperPhysics***** Quantum Physics | R Nave |