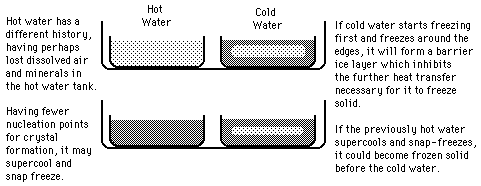
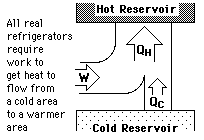
Hot Water Freezing?
The rumor persists that hot water will freeze faster than cold water. It sounds implausible because the hot water has more internal energy which must be removed before it can start the phase change. Perhaps this tale had its origin in the days of non-frost-free refrigerators in which a considerable buildup of ice on the walls of the freezing compartment was a common occurrence. Ice acts as an insulator, inhibiting the cooling process.
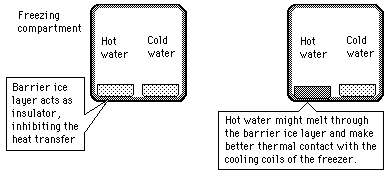
| Another scenario | The Mpemba effect |
Heat questions