
    |
    |
| Heat |

|
Index | |||
| HyperPhysics***** Thermodynamics | Go Back |
Water&Ice, Water&SteamHow can so much energy be put into a substance during a phase change without changing its temperature?
The key idea is that temperature does not measure the entire internal energy of a substance, only the translational kinetic energy part. During a phase change, the energy goes into the potential energy part, either taking from or adding to the energy associated with the molecular attractions. 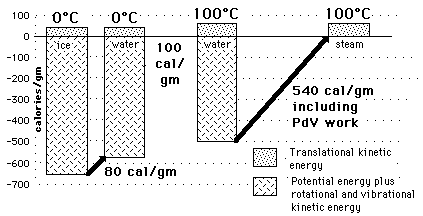
|
Index Internal energy concepts Heat questions | ||||
| HyperPhysics***** Thermodynamics | Go Back |
Water and Ice at 0 CThe constancy of temperature when water is going through its phase changes at 0 C and 100 C provides some insight into the nature of the internal energy of water, ice, and steam and into the nature of temperature. The fact that large amounts of energy are added to accomplish melting or vaporization without a change in temperature shows that temperature does not measure all of the internal energy of the water - in fact it is only a measure of the translational kinetic energy of the water molecules. The large size of the phase change energy indicates that there is a large amount of potential energy associated with the intermolecular forces between the water molecules. During the melting and vaporization phase changes, the energy goes into the breaking down of the solid or liquid bonds, reducing the binding potential energy by the amount of the phase change energy and therefore not changing the average translational kinetic energy as reflected by the temperature. |
Index Internal energy concepts Heat questions |
| HyperPhysics***** Thermodynamics | Go Back |
Steel and Wood at 0 CYour sense of touch is not a reliable thermometer because the sense of "hotness" or "coldness" of a surface depend upon other factors as well as the temperature. Most notably, the thermal conductivity of the object touched plays a part in how hot or cold it feels. With both objects at 0íC, steel feels colder than wood because its thermal conductivity is much higher and it conducts energy away from your finger faster. If both objects are at 100íC, much higher than body temperature, the steel will feel hotter than the wood because it will conduct energy to your finger much more rapidly. |
Index Internal energy concepts Heat questions |
| HyperPhysics***** Thermodynamics | Go Back |