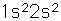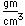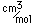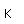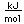Beryllium
Beryllium is a light, silvery-white metal. It is used for making windows for x-ray tubes and radiation counters. X-rays readily penetrate materials of low atomic number, and beryllium metal has the best mechanical properties for the production of such windows.
Beryllium is also used in specialty alloys. About 2% of beryllium in copper produces a hard alloy used for making springs.
The principal ore of beryllium is beryl, Be3Al2(SiO3)6. Gem varieties of beryl include emerald, which is beryl with traces of chromium to contribute the green color. Also of gem quality is trapiche, or trapiche emerald, which is a green gem with a marked spoke pattern. Aquamarine is also a bluegreen variety of beryl. Beryllium is found in the phosphate minerals Beryllonite, Herderite and roscherite.
Beryllium forms the silicate phenakite, Be2SiO4.
Beryllium is also found in the silicate mineral Bertrandite. Other silicates of beryllium are leucophanite, helvite and milarite. It is found with yttrium in the silicate mineral gadolinite. Another silicate mineral of beryllium is euclase. A silicate of beryllium with sodium and aluminum is tugtupite. A silicate of beryllium with sodium is epididymite.
With aluminum, beryllium forms the oxide mineral Chrysoberyl including a gem-quality variety called Alexandrite. With boron, beryllium forms the oxide mineral Hambergite. With magnesium and aluminum, beryllium forms the oxide mineral taafeite.
Compounds of beryllium are very poisonous - dust or powder from the metal or its oxides can cause serious illness.

|