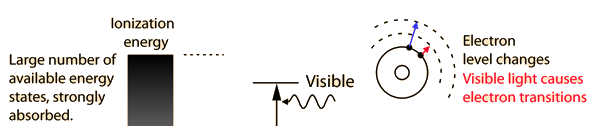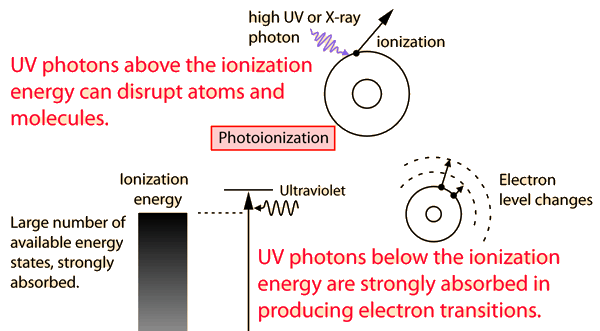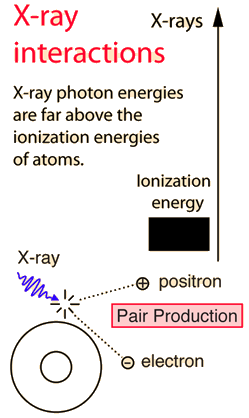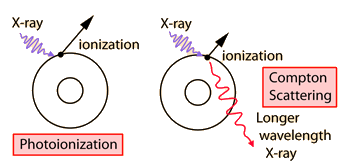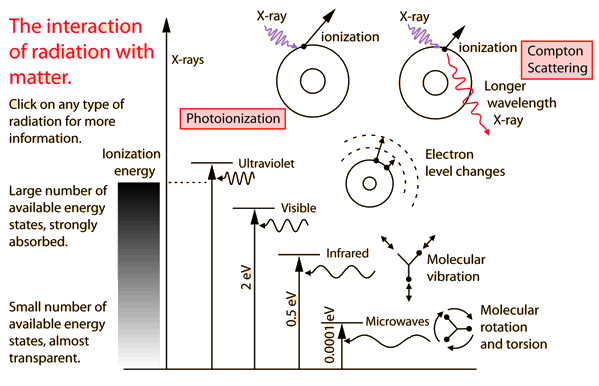
You may click on any of the types of radiation for more detail about its particular type of interaction with matter. The different parts of the electromagnetic spectrum have very different effects upon interaction with matter. Starting with low frequency radio waves, the human body is quite transparent. (You can listen to your portable radio inside your home since the waves pass freely through the walls of your house and even through the person beside you!) As you move upward through microwaves and infrared to visible light, you absorb more and more strongly. In the lower ultraviolet range, all the uv from the sun is absorbed in a thin outer layer of your skin. As you move further up into the x-ray region of the spectrum, you become transparent again, because most of the mechanisms for absorption are gone. You then absorb only a small fraction of the radiation, but that absorption involves the more violent ionization events. Each portion of the electromagnetic spectrum has quantum energies appropriate for the excitation of certain types of physical processes. The energy levels for all physical processes at the atomic and molecular levels are quantized, and if there are no available quantized energy levels with spacings which match the quantum energy of the incident radiation, then the material will be transparent to that radiation, and it will pass through. If electromagnetic energy is absorbed, but cannot eject electrons from the atoms of the material, then it is classified as non-ionizing radiation, and will typically just heat the material.
Electromagnetic spectrum annotated with physiological effects
| HyperPhysics***** Quantum Physics | R Nave |