Light Emitting Diode Structure
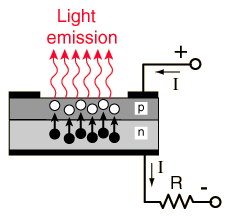 |
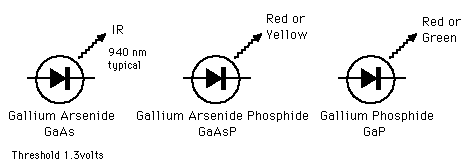
LEDs are p-n junction devices constructed of gallium arsenide (GaAs), gallium arsenide phosphide (GaAsP), or gallium phosphide (GaP). Silicon and germanium are not suitable because those junctions produce heat and no appreciable IR or visible light. The junction in a LED is forward biased and when electrons cross the junction from the n- to the p-type material, the electron-hole recombination process produces some photons in the IR or visible in a process called electroluminescence. An exposed semiconductor surface can then emit light. |
| Circuit symbol | Packaging | Characteristics |
| More detailed structure of device |
LED concepts
Reference
Floyd
Electronic Devices, Sec. 3-4
| HyperPhysics*****Electricity and magnetism***** Quantum Physics ***** Optics | R Nave |