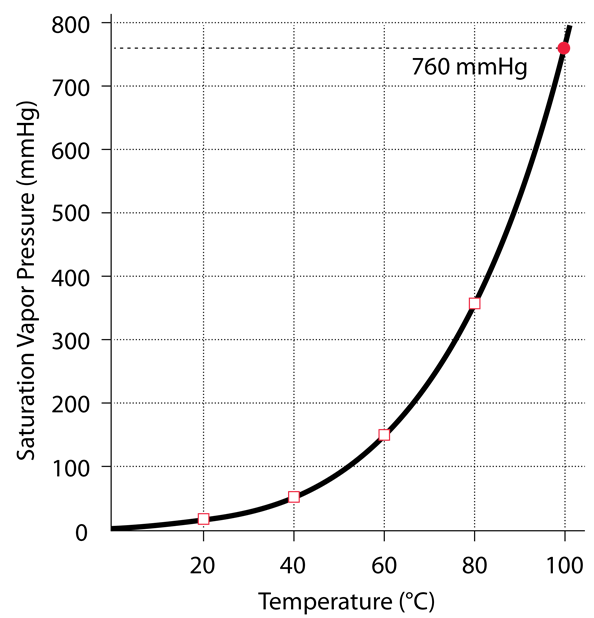
Saturated Vapor Pressure for Water
| Saturation vapor pressure | Table for water | Vapor density graph |
Kinetic theory concepts
Applications of kinetic theory
Vapor application concepts
| HyperPhysics***** Thermodynamics | R Nave |
Saturated Vapor Pressure, Density for Water
Below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. The pressures are stated in mega-Pascals, where a Pascal is a Newton per square meter, and as a multiple of standard atmospheric pressure.
|
Index Kinetic theory concepts Applications of kinetic theory Vapor application concepts | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Go Back |
Saturated Vapor Pressure for Water

|
Index Kinetic theory concepts Applications of kinetic theory Vapor application concepts | |||
|
Go Back |