PvT Surface for a Substance which Expands Upon Freezing
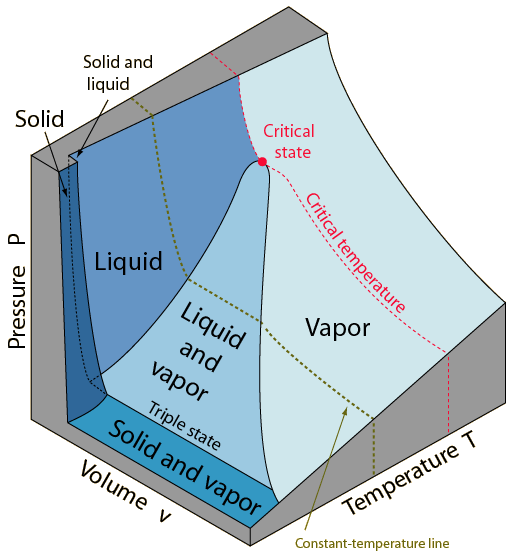
The equilibrium states of a simple, compressible substance can be specified in terms of its pressure, volume and temperature. If any two of these state variables is specified, the third is determined. This implies that the states of the substance can be represented as a surface in a three dimensional PvT space. The PvT surface above represents a substance which expands upon freezing. The vast majority of substances contract upon freezing. The notable exception is water for which the expansion upon freezing has an enormous impact on the nature of the Earth. A considerable amount of information about the phases of matter can be illustrated with the PvT surface.
The solid, liquid and gas(vapor) phases can be represented by regions on the surface. Note that there are regions on the surface which represent a single phase, and regions which are combinations of two phases. A point lying on a line between a single-phase and a two-phase region represents a "saturation state". The line between the liquid and the liquid-vapor regions is called the liquid-saturation line and any point on that line represents a saturated-liquid state. A point on the boundary between the vapor and the liquid-vapor regions is called a saturated-vapor state.
Note the critical state where the saturated-liquid and saturated-vapor lines meet. The state variables of this unique point are denoted by Pc, vc and Tc. If a substance is above the critical temperature Tc, it cannot condense into a liquid, no matter how high the pressure. This merging of the liquid and vapor states above the critical temperature is a characteristic of all known substances. While a pure vapor state can exist at a pressure lower than Pc, at pressures above Pc it is constrained to be a vapor. States with pressures above Pc are described as "supercritical states".
The remarkable "triple state" of matter where solid, liquid and vapor are in equilibrium may be characterized by a temperature called the triple point. The triple state is represented by a line parallel to the Pv plane with a characteristic pressure for the substance but variable volume. The triple point temperature of water is assigned the value 273.16 K and the triple state of water is used as the reference for establishing the Kelvin temperature scale.
| Table of triple-state data |
Reference
Wark and Richards
Ch 3
| HyperPhysics***** Thermodynamics | R Nave |