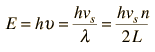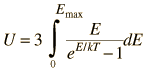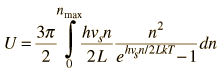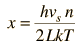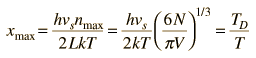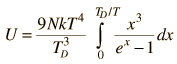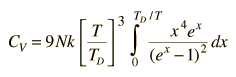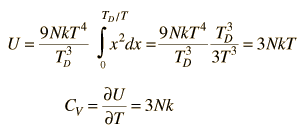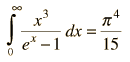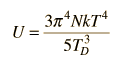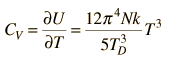What is a Phonon?
Considering the regular lattice of atoms in a uniform solid material, you would expect there to be energy associated with the vibrations of these atoms. But they are tied together with bonds, so they can't vibrate independently. The vibrations take the form of collective modes which propagate through the material. Such propagating lattice vibrations can be considered to be sound waves, and their propagation speed is the speed of sound in the material.
The vibrational energies of molecules, e.g., a diatomic molecule, are quantized and treated as quantum harmonic oscillators. Quantum harmonic oscillators have equally spaced energy levels with separation ΔE = hυ. So the oscillators can accept or lose energy only in discrete units of energy hυ.
The evidence on the behavior of vibrational energy in periodic solids is that the collective vibrational modes can accept energy only in discrete amounts, and these quanta of energy have been labeled "phonons". Like the photons of electromagnetic energy, they obey Bose-Einstein statistics.
Considering a solid to be a periodic array of mass points, there are constraints on both the minimum and maximum wavelength associated with a vibrational mode.
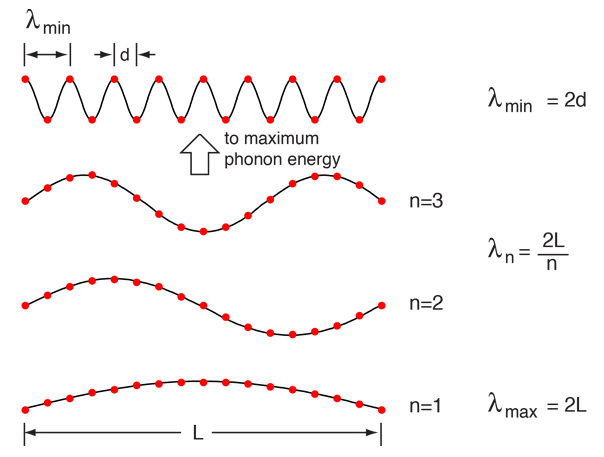
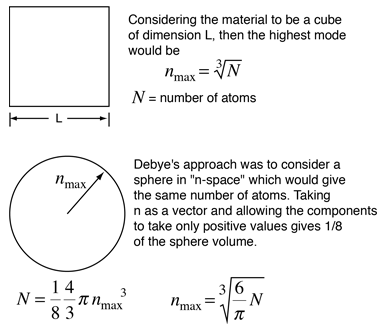 |
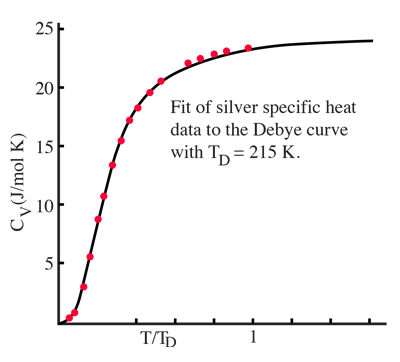
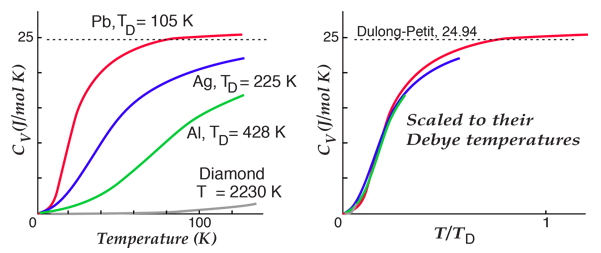
By associating a phonon energy 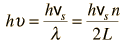 with the modes and summing over the modes, Debye was able to find an expression for the energy as a function of temperature and derive an expression for the specific heat of the solid. In this expression, vs is the speed of sound in the solid. |
| Debye specific heat |
Reference
Schroeder
Ch 7.
| HyperPhysics***** Thermodynamics | R Nave |