The Correspondence Principle and the Quantum Oscillator
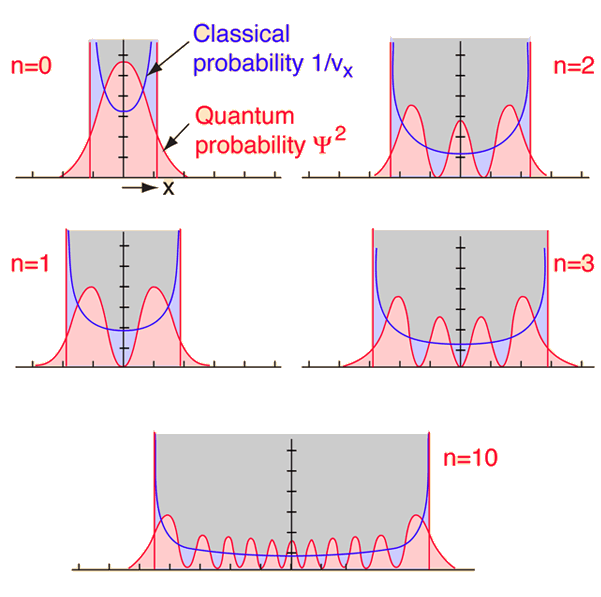
Quantum mechanics is necessary for the description of nature on the atomic scale, but Newton's laws do fine for baseballs. Somewhere along the continuum from quantum to classical, the two descriptions must merge. Starting from the quantum end and noting that energies depend upon some quantum number, one would anticipate that for high enough quantum numbers, the quantum treatment should merge with the classical. This idea of the merging of the quantum and classical is called the "correspondence principle"
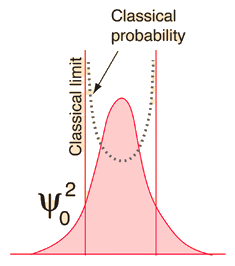 | If you examine the ground state of the quantum harmonic oscillator, the correspondence principle seems far-fetched, since the classical and quantum predictions for the most probable location are in total contradiction. If the equilibrium position for the oscillator is taken to be x=0, then the quantum oscillator predicts that for the ground state, the oscillator will spend most of its time near that center point. The classical mass-on-a-spring will obviously spend very little time there because it has the maximum speed there; it will spend most of its time near the end points of its oscillation where its velocity is small. |
As you go to higher and higher states of the quantum oscillator by increasing n, the most probable location shifts to the outside of the well. You still have the undulation of the probability which is characteristic of the wave solution, but at least the overall trend of the probability begins to look more like the classical probability which is shown by the dashed line.
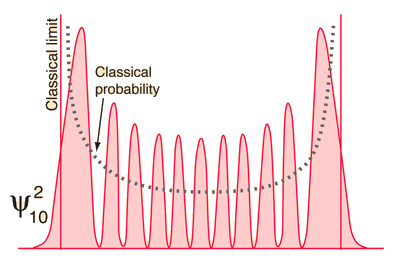 | This wild oscillation of the probability has practical consequences only if it is observable, and the higher the quantum number, the smaller the distance between peaks. At high enough n, the uncertainty principle will prevent you from resolving the gaps without altering the physical state of the oscillator. The exponential tails into the classically forbidden region decrease with increasing n, so the quantum oscillator looks more and more like the classical one. |
Bohr used the term "correspondence principle" and expected that the radiative behavior of atoms would approach the classical radiation from accelerated charges for high enough quantum states. Certainly the observed line spectra from atoms differ radically from classical behavior. Beiser gives an example of calculating the radiation frequency of an atom for quantum number n=10,000 and states that it differs from the classical result by only 0.01%.
| Further detail about classical and quantum probabilities |
Schrodinger equation concepts
References
Beiser, Perspectives
Sec 6-6
Thornton & Rex
Sec 7-6, 8-7
| HyperPhysics***** Quantum Physics | R Nave |