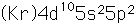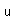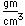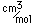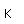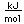Tin
Tin is a silvery-white metal with great malleability, permitting it to be hammered or rolled into thin sheets called tin-foil. Ordinary white tin has metallic properties, but at temperatures below 18°C it slowly changes form to a non-metallic allotropic modification, gray tin, which has the diamond structure. At very low temperatures, <-50°C, the speed of conversion may be fast enough for metallic tin objects to disintegrate into a powder of gray tin.
Tin finds extensive use as a protective layer for mild steel. Tin plating is done by dipping clean sheets of mild steel into molten tin, or by electroplating. Copper is also sometimes clad with tin in this way.
The principal alloys of tin are bronze (tin and copper), soft solder (tin and lead), pewter (75% tin and 25% lead), and britannia metal (tin with small amounts of antimony and copper).
Alloys of tin, lead, antimony and copper find application in surface contact bearings. They contain small hard crystals of a compound such as SnSb embedded in a soft matrix of tin or lead. The good bearing properties result from orientation of the hard crystals to present flat surfaces at the bearing surface.
Tin oxide, SnO2, is found in the mineral form cassiterite. It appears with iron and magnesium in the mineral Hulsite. Tin forms a sulfide with lead, iron and antimony is called cylindrite. A sulfide formed with lead and antimony is called franckeite. Stannite is a sulfide with tin and copper. Tin appears with calcium in the silicate stokesite.
|