Hemoglobin and Myoglobin
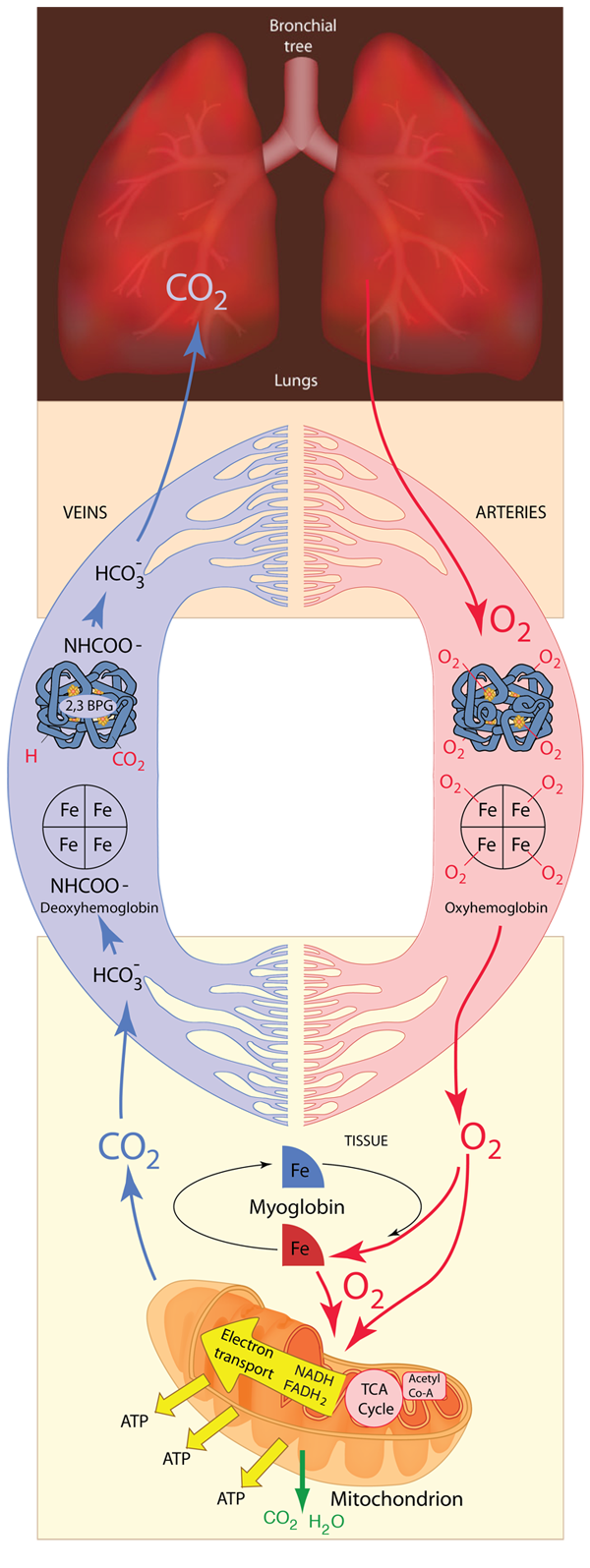
The respiratory system must provide a continuous supply of oxygen to all parts of the body. The oxygen is necessary for metabolism throughout the body and the final destination in its journey is in the process of providing energy in the form of ATP for all parts of the body's processes. As shown below, the process that begins in the lungs makes use of a transport protein called hemoglobin to transport the oxygen to the tissue, and also makes extensive use of another protein, myoglobin, for the storage of energy. The hemoglobin is carried in the blood supply by red blood cells. Oxygen is transported to the mitochondria in cells where the electron transport chain finally places the oxygen in water molecules to be exhaled.
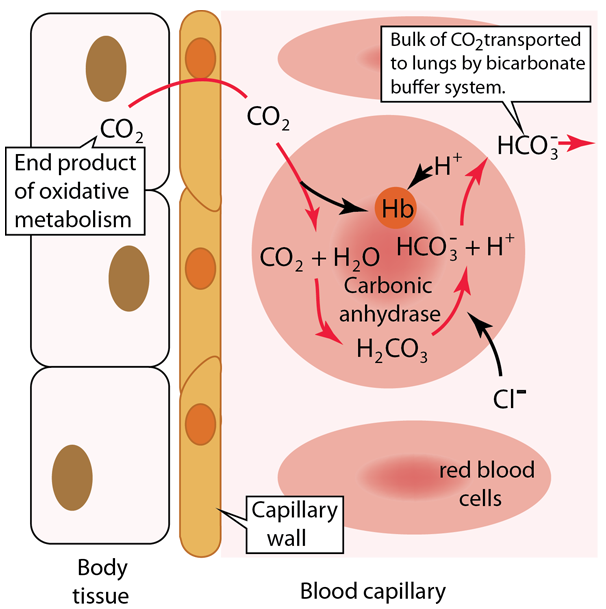
| Carbon Dioxide Transport to Lungs |
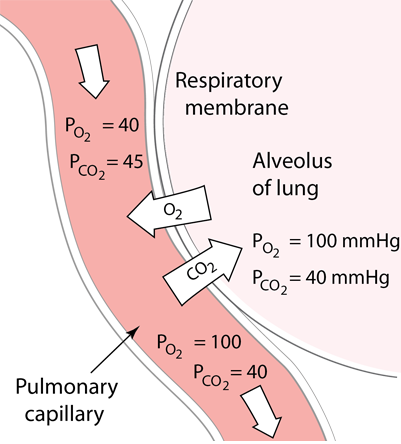 | Oxygen in the lungs diffuses into the pulmonary capillaries and into red blood cells to bind to hemoglobin. This diffusion process of gas exchange depends upon the partial pressures and the solubility in the fluids and is described in more detail in Fick's Law, Graham's Law and Henry's Law At the same time as the oxygen is preferentially diffusing into the blood, there is net diffusion of CO2 from the blood into the lungs to be exhaled. |
Hemoglobin is an essential protein in the body, performing the major part of the oxygen transport in the blood. Hemoglobin contains iron which gives it a red color, and it contributes that color to the red blood cells and the blood itself.
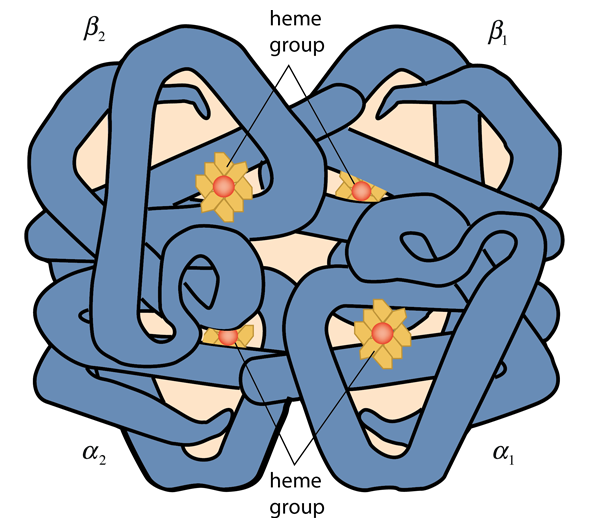
Hemoglobin consists of two types of components call heme and globin. Globin is a protein of 574 amino acids in four polypeptide chains. Each of those chains is associated with a heme group. Each heme group surrounds an atom of iron, and each iron atom can loosely bind an oxygen atom. Binding up to four oxygen molecules, the hemoglobin forms the compound called oxyhemoglobin. Myoglobin consists of one polypeptide chain similar to one of the four in the structure above, and can bind only one oxygen molecule. It is abundant in muscle cells and acts as a storage location for oxygen which the cell calls upon in times of low oxygen supply.
Any animal greater than a few millimeters in size must ensure a steady supply of oxygen to cells throughout its body and remove waste products such as carbon dioxide. In all vertebrates and even in some microscopic life the transport protein is hemoglobin. Myoglobin is also used by most animals in muscle tissue to provide an oxygen reserve for periods of high oxygen demand.
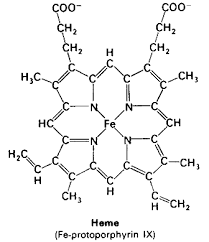 | An iron atom in the state FeII forms an iron porphyrin which provides the vivid red color for blood and red blood cells. Plants use a magnesium porphyrin in chlorophyll which gives plants their green color. This iron porphyrin binds to the proteins to provide the oxygen binding site. |
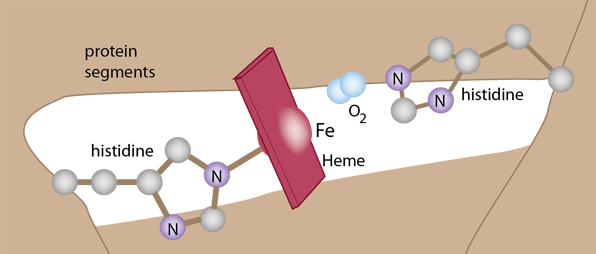
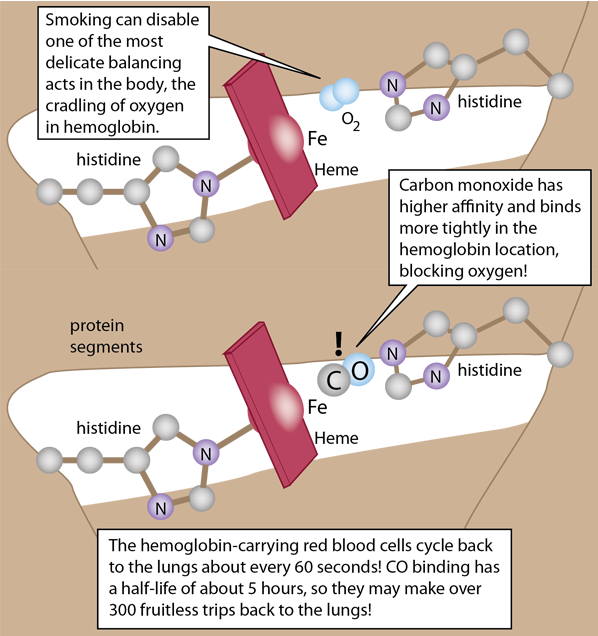
The details of the polypeptide environment of both myoglobin and hemoglobin bind the oxygen but protect the iron from oxidation. This exquisitely balanced environment allows the oxygen to bind and be released so that it can bind another oxygen. The framework for holding the heme involves two histidines which help stabilize the heme in a pocket of the globular structure of either myoglobin or hemoglobin.
The heme pocket is ideal for oxygen, but carbon monoxide actually binds to both hemoglobin and myoglobin with much greater affinity and that binding is not readily reversible. This makes CO a potent poison in the body.
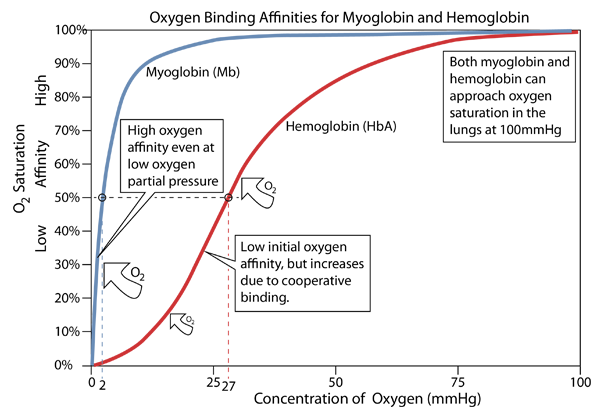
When provided with a supply of oxygen, myoglobin will bind oxygen rapidly even at very low partial pressures of oxygen. That makes it efficient as a storage location for oxygen, but this kind of affinity curve makes it a poor supplier of oxygen at typical partial pressure values in tissue. Hemoglobin's affinity starts low and rises gradually up to a supply partial pressure of about 20 mmHg, but then turns upward sharply. This change in its behavior (an allosteric effect) comes from the fact that the binding of an oxygen molecule to the first of the four hemes causes a configuration change in hemoglobin that makes it easier for it to bind oxygen to the other three hemes. This is called "cooperative binding" and it is of tremendous importance in the efficiency of oxygen transport in animals.
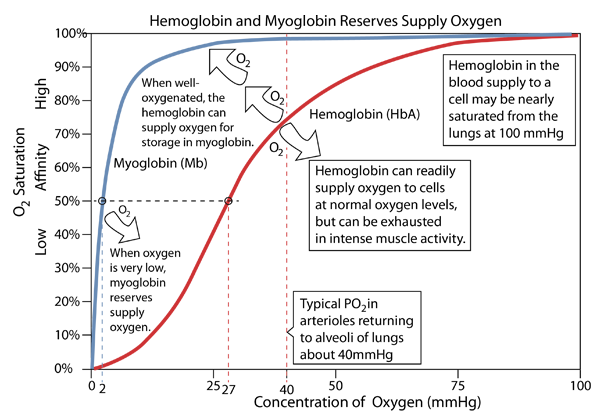
Myoglobin in tissue - especially in muscle tissue - accepts oxygen from hemoglobin and stores it. It can then deliver the oxygen to the mitochondria when their oxygen needs are sufficiently great.
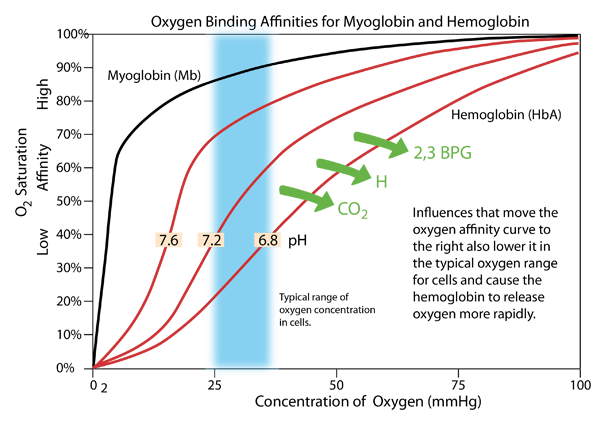
The pH of the blood is close to the neutral pH of 7.4, typically ranging from 7.35-7.45 and needing to be maintained in that range. But inside cells the pH can vary over a wider range, and the pH affects the oxygen supply process. If the pH becomes lower (more acidic), the oxygen affinity curve of hemoglobin moves to the right as shown above, and exhibits lower affinity in the range of oxygen concentration typical of the cellular interior. Active metabolism in the cell produces CO2 and H, which act to lower the pH. This causes oxygen to be released more rapidly in an effect known as the Bohr Effect, named after Christian Bohr who discovered this phenomenon.
An additional effect is the binding of the compound 2,3 bisphosphoglycerate (2,3 BPG) which enhances the stability of the deoxyhemoglobin and also has the affect of moving the affinity curve to the right, enhancing oxygen release. |
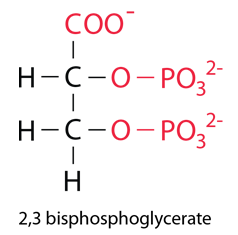 |
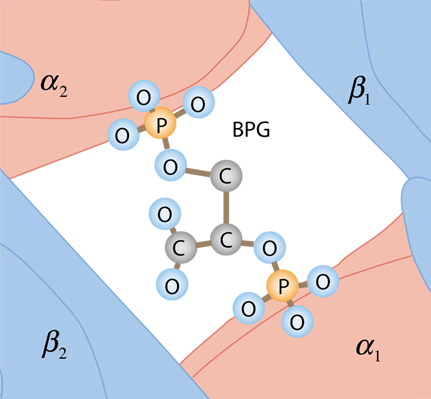 | The BPG is bound in the center space between the four globulins of deoxyhemoglobin by the surface charges on the proteins. It does not fit in this space for oxyhemoglobin, so it does not bind. |
Biochemical concepts
Chemistry concepts
Reference
Shipman, Wilson and Todd
Ch 15
Matthews, van Holde, Ahern
Ch 7
Ahern
Ch 6
| HyperPhysics*****Chemistry | R Nave |