Broadening of Spectral Lines
In the study of transitions in atomic spectra, and indeed in any type of spectroscopy, one must be aware that those transitions are not precisely "sharp". There is always a finite width to the observed spectral lines.
One source of broadening is the "natural line width" which arises from the uncertainty in energy of the states involved in the transition. This source of broadening is important in nuclear spectra, such as Mossbauer spectra, but is rarely significant in atomic spectroscopy. A typical lifetime for an atomic energy state is about 10-8 seconds, corresponding to a natural linewidth of about 6.6 x 10-8 eV.
For atomic spectra in the visible and uv, the limit on resolution is often set by Doppler broadening. With the thermal motion of the atoms, those atoms traveling toward the detector with a velocity v will have transition frequencies which differ from those of atoms at rest by the Doppler shift. The distribution of velocities can be found from the Boltzmann distribution.
Since the thermal velocities are non-relativistic, the Doppler shift in the angular frequency is given by the simple form
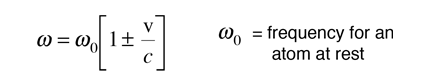
From the Boltzmann distribution, the number of atoms with velocity v in the direction of the observed light is given by
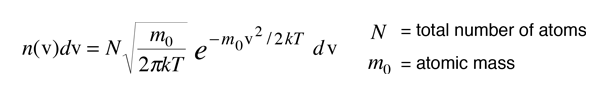
The distribution of radiation around the center frequency is then given by
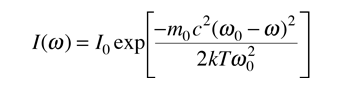
This is in the form of a Gaussian, and the width at half-maximum is given by
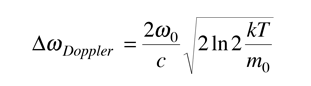
Often it is convenient to express this in terms of wavelength.
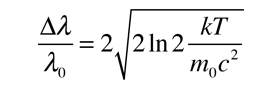
When you move further down the spectrum into the microwave region for molecular rotational spectra, the natural linewidth again emerges as a larger source of broadening than Doppler broadening. At some pressure, the perturbations of rotational energy levels by molecular collisions (pressure broadening) becomes the limiting factor for resolution.
| Thwarting Doppler broadening with saturation spectroscopy |
Atomic Structure Concepts
Reference
Haken & Wolf
Sec 16.2
| HyperPhysics***** Quantum Physics | R Nave |