Auger Effect
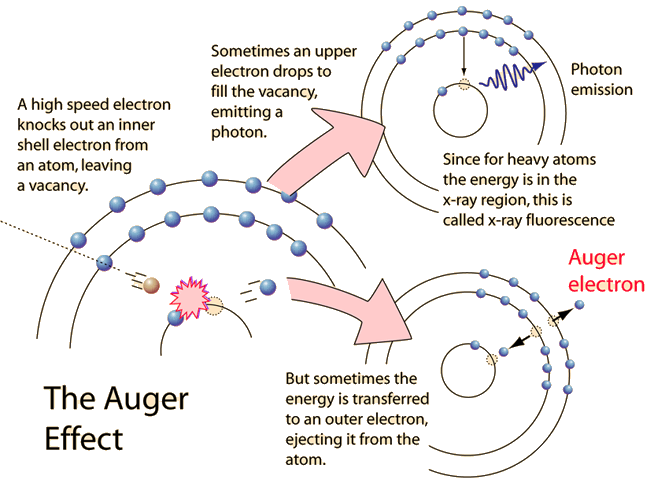
The Auger effect is a process by which electrons with characteristic energies are ejected from atoms in response to a downward transition by another electron in the atom. In Auger spectroscopy, the vacancy is produced by bombardment with high energy electrons, but the Auger effect can occur if the vacancy is produced by other interactions. It is observed as one of the methods of electron rearrangement after electron capture into the nucleus.
The diagram of Auger electron emission above is over-simplified because the energy transferred to the outer electron to eject it would not in general be transferred entirely to that electron, but could also produce a photon along with the ejected electron. Taking as an example a transition that produced a Kα x-ray, the illustration at right shows the energy of the Kα x-ray photon divided between the kinetic energy of the Auger electron and an emitted photon after overcoming the electron binding energy U. | 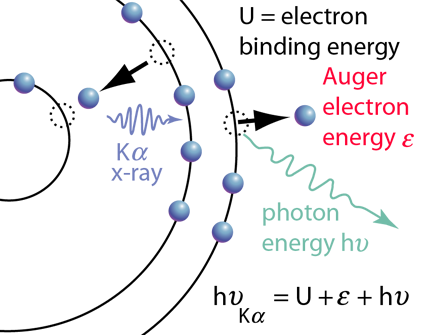 |
If an inner shell electron is removed from an atom, an electron from a higher level will quickly make the transition downward to fill the vacancy. Sometimes this transition will be accompanied by an emitted photon whose quantum energy matches the energy gap between the upper and lower level. Since for heavy atoms this quantum energy will be in the x-ray region, it is commonly called x-ray fluorescence. This emission process for lighter atoms and outer electrons gives rise to line spectra.
In other cases, the energy released by the downward transition is given to one of the outer electrons instead of to a photon, and this electron is then ejected from the atom with an energy equal to the energy lost by the electron which made the downward transition minus the binding energy of the electron that is ejected from the atom. Though more involved in interpretation than optical spectra, the analysis of the energy spectrum of these emitted electrons does give information about the atomic energy levels. The Auger effect bears some resemblance to internal conversion of the nucleus, which also ejects an electron.
| Auger spectrum of a surface |
Atomic Structure Concepts
| HyperPhysics***** Quantum Physics | R Nave |