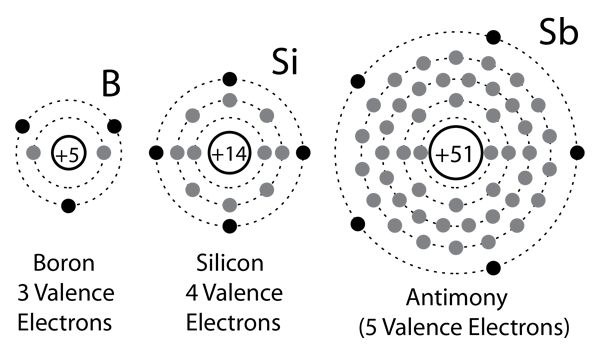
Silicon and Germanium
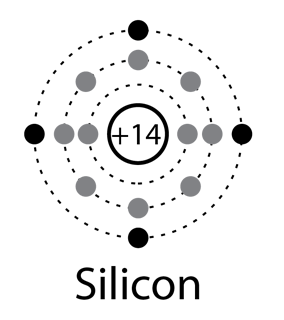
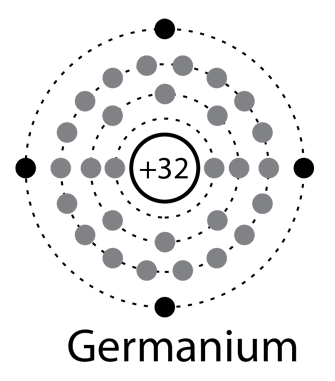
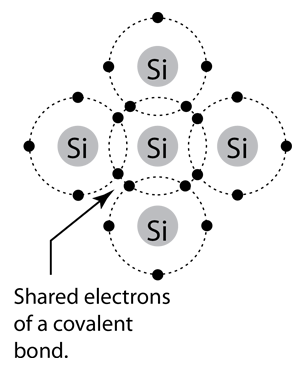
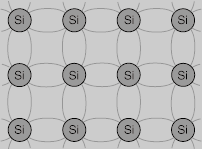
Solid state electronics arises from the unique properties of silicon and germanium, each of which has four valence electrons and which form crystal lattices in which substituted atoms (dopants) can dramatically change the electrical properties.
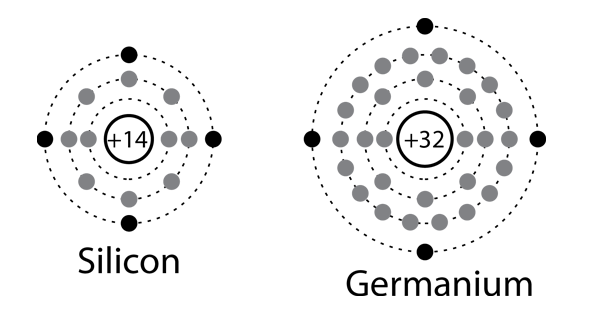
Semiconductor concepts
| HyperPhysics***** Condensed Matter | R Nave |