Thermodynamic Potentials
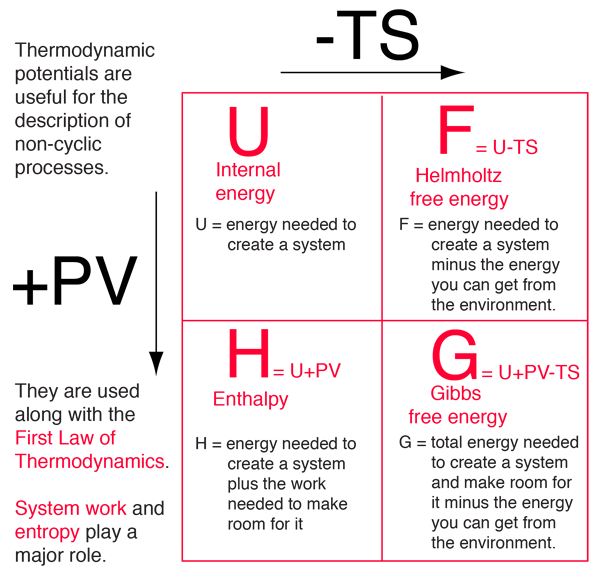
Four quantities called "thermodynamic potentials" are useful in the chemical thermodynamics of reactions and non-cyclic processes. They are internal energy, the enthalpy, the Helmholtz free energy and the Gibbs free energy.
|
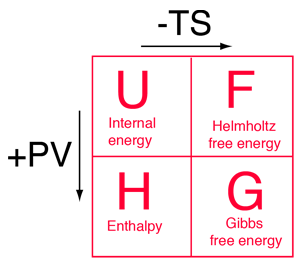
The four thermodynamic potentials are related by offsets of the "energy from the environment" term TS and the "expansion work" term PV. A mnemonic diagram suggested by Schroeder can help you keep track of the relationships between the four thermodynamic potentials. |
 |
Internal energy concepts
Reference
Schroeder
Ch 5
| HyperPhysics***** Thermodynamics | R Nave |