Of Molecules and Baseballs
The motion of baseballs can reasonably be described by Newton's laws, but the motion of molecules in a gas cannot. It's not that Newton's laws are not as good for gas molecules as baseballs; it's just that we can't track Avogadro's number of them and are forced to use averages or other statistical properties of the collection of molecules. While that might seem to doom us to inaccurate and erratic descriptions of nature, that is definitely not the case. The statistics of very large numbers of events gives a precise description of the collective behavior of the particles.
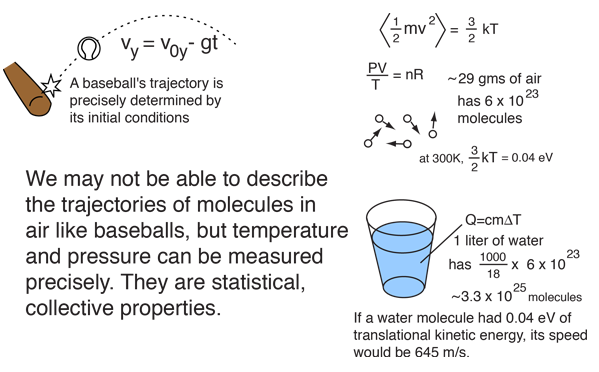
The field of thermodynamics involves descriptions of phenomena which are too small for us to see. These descriptions are statistical in nature in that they deal with averages over vast numbers of particles. This involves the concepts of internal energy and temperature. It yields relationships like the ideal gas law, equipartition of energy, and specific heat which allow us to make precise predictions about the collective behavior of matter. You can't precisely describe a single molecule like you can a baseball, but the collective behavior of the group is well determined.
| Temperature and kinetic energy | Equipartition of energy | Thermal energy |
Internal energy concepts
| HyperPhysics***** Thermodynamics | R Nave |