Liquifying Air
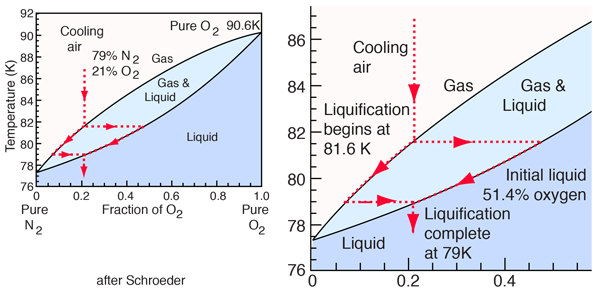
The liquification of air is an interesting process since it's composition is mainly a mixture of two diatomic gases, 78% N2 and 21% O2. Daniel Schroeder gives an excellent description of this process. The illustration above is adapted from his summary of data from the National Research Council's International Critical Tables, Volume 3 and from the CRC Handbook (Ed. Lide). The initial oxygen concentration of the liquid has been revised to 51.4% based on more recent measurements.
The liquification point of a mixture of gases with different boiling points will typically be between the boiling points of the pure gases. The boiling point of pure oxygen, O2, is 90.6 K and the boiling point of nitrogen, N2, is 77 K. If air is cooled at atmospheric pressure, it remains a gas until the temperature reaches 81.6 K, 9 K below the liquification temperature of pure oxygen. It will be completely liquified at about 79 K, 2K above the liquification point of pure nitrogen. Plunging an air-filled balloon into liquid nitrogen will lead to the liquification of almost all the air in the balloon. The liquid can be clearly seen inside the balloon.
Typical of the balloons used in a "shrinking balloon" demonstration is an air volume of about 6 liters or about one quarter of a mole. Using 29 grams as a mole of air and assuming a liquid density of 0.785 gm/cm3 leads to an estimate of almost 10 cm3 of liquid from such a balloon.
| Go to movie showing condensed liquid |
Temperature concepts
Liquid nitrogen
References
Schroeder
Ch 5
Lide
| HyperPhysics***** Thermodynamics | R Nave |