The 14 Bravais Lattices
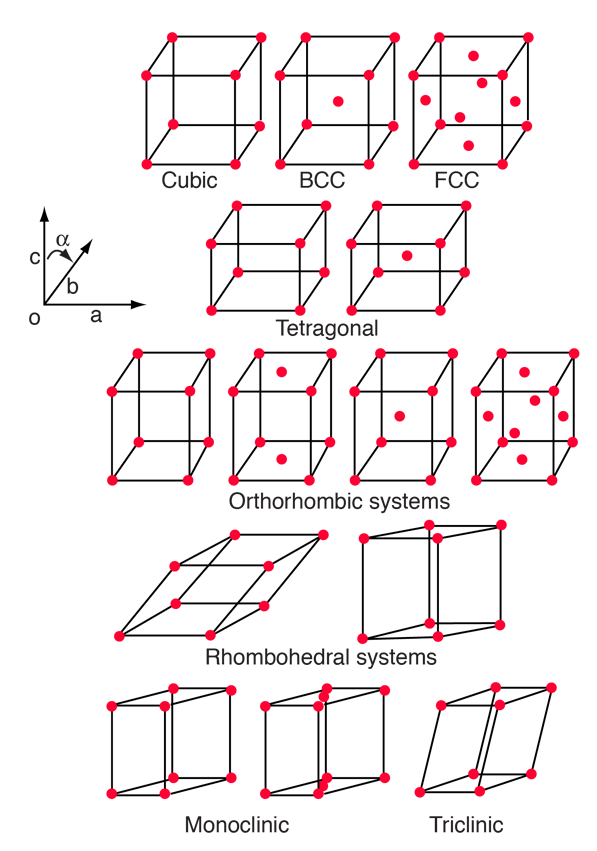
Most solids have periodic arrays of atoms which form what we call a crystal lattice. Amorphous solids and glasses are exceptions. The existence of the crystal lattice implies a degree of symmetry in the arrangement of the lattice, and the existing symmetries have been studied extensively. A crystal structure is one of the characteristics of minerals.
One of the implications of the symmetric lattice of atoms is that it can support resonant lattice vibration modes. These vibrations transport energy and are important in the thermal conductivity of non-metals, and in the heat capacity of all solids.
References
Myers
Ch 2
Kittel, Intro to Solid State
Ch 1
| HyperPhysics***** Condensed Matter ***** Electricity and Magnetism | R Nave |