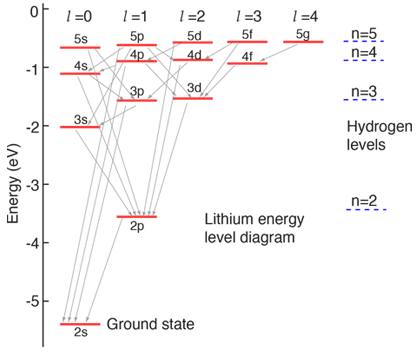
Lithium Energy Levels
|
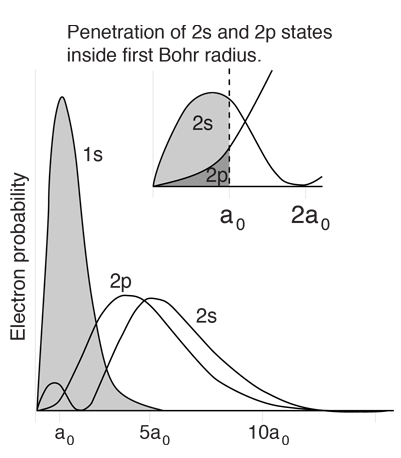
The lithium atom has a closed n=1 shell with two electrons and then one electron outside. Since the outer electron looks inward at just one net positive charge, it could be expected to have energy levels close to those of hydrogen. This is true for high angular momentum states as shown, but the s and p states fall well below the corresponding hydrogen energy levels.
|
The general layout here was taken from Rohlf but some discrepancies were found in the lower levels. The levels up through the 3d plus the 4p and 4d were rescaled to fit the data in the table of neutral lithium levels from NIST. Discrepancies may remain with the other levels. The ionization potential given by NIST was 5.395 eV.
|
Index
Rohlf Sec 9.5, Fig 9.9 |