Bragg's Law
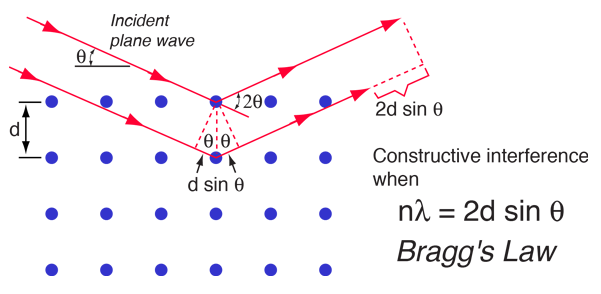
When x-rays are scattered from a crystal lattice, peaks of scattered intensity are observed which correspond to the following conditions:
- The angle of incidence = angle of scattering.
- The pathlength difference is equal to an integer number of wavelengths.
The condition for maximum intensity contained in Bragg's law above allow us to calculate details about the crystal structure, or if the crystal structure is known, to determine the wavelength of the x-rays incident upon the crystal.
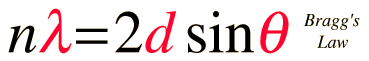
This calculation is designed to calculate wavelength, crystal plane separation or diffraction angle. After entering data, click on the symbol of the quantity you wish to calculate in the active graphic above. Default data will be entered for any unspecified quantity, but all values can be changed.
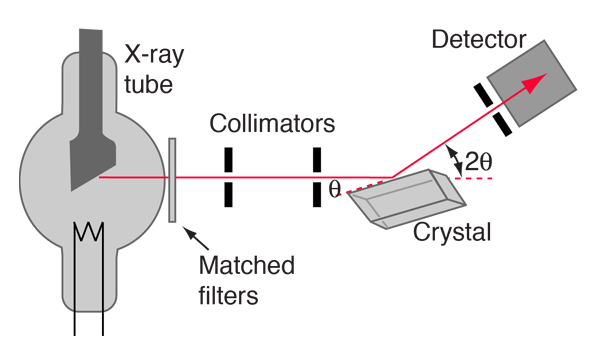
| Bragg spectrometer | Characteristic x-rays |
| Brillouin scattering: application to light scattering |
Reference
Thornton & Rex
Sec. 6.1
| HyperPhysics***** Quantum Physics | R Nave |