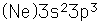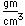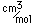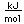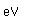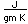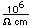Phosphorus
Phosphorus is a low melting-point non-metal which occurs in several forms. Phosphorus vapor just above its boiling point of 280.5°C consists of tetratomic molecules in a tetrahedral form in contrast to its higher temperature form with is diatomic and similar to the nitrogen diatomic form. This tetratomic vapor condenses to liquid white phosphorus which freezes at 44.1°C to solid white phosphorus, a soft, waxy, colorless material. In this solid form it maintains its P4 molecules.
White phosphorus is metastable and slowly changes to a stable form, red phosporus, in the presence of light or upon heating. Red phosphorus is far more stable than the white form. It does not catch fire in air at temperatures below 240°C whereas white phosphorus ignites at about 40°C. Red phosphorus cannot be converted into white phosphorus except by vaporizing it.
White phosphorus is very poisonous, a lethal dose being about 0.15 grams. It causes necrosis of the bones, especially those of the jaw. Red phosphorus is not poisonous.
Phosphorus in nature occurs as phosphate minerals including fluorapatite, Ca5(PO4)3F, hydroxy-apatite, Ca5(PO4)3(OH) and tricalcium phosphate Ca3(PO4)2. Hydroxyapatite is the main constituent of teeth and bones. Complex organic compounds of phosphorus are essential constituents of nerve and brain tissue and of many proteins, and are involved in the metabolic reactions of living organisms.
Large amounts or phosphorus are converted to phosphoric acid.
Phosphorus is used in the making of matches. The older practice of using white phosphorus for matches has been abandoned because of the extremely poisonous nature of white phosphorus. Ordinary matches are made by dipping the end of the match into paraffin, then into a wet mixture of phosphorus sulfide, P4S3, lead dioxide or other oxidizing agent, and glue. The heads of safety matches contain antimony trisulfide and potassium chlorate or dichromate, and the striking strip on the box is coated with red phosphorus, powdered glass, and glue.
Phosphorus forms the hydride PH3 which is called phosphine. It is an exceedingly poisonous gas.
Phosphates are valuable fertilizers, and the annual use of phosphate rock for this purpose is in the tens of millions of tons. It must be converted to calcium dihydrogen phosphate (Ca(H2PO4)2) to be assimilable by plants.
Common phosphorus has a nominal density of 1.83 gm/cm3, but in the white form has a density of 5.7 and in the red form 5.1.
|