Metals and Nonmetals
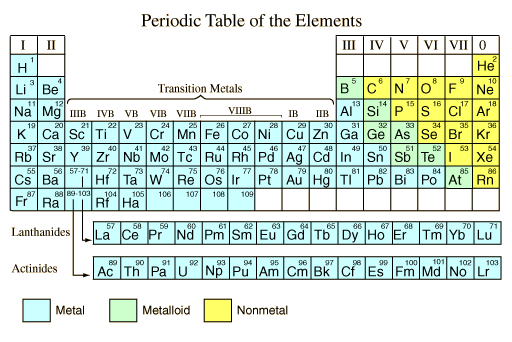
As shown on the periodic table of the elements below, the majority of the chemical elements in pure form are classified as metals. It seems appropriate to describe what is meant by "metal" in general terms. This general description is adapted from Shipman, et al.
|
|
|
|
| Lewis dot diagrams of elements |
Chemical concepts
Reference
Shipman, Wilson, Todd
Ch 12
| HyperPhysics***** Quantum Physics | R Nave |