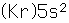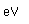Strontium
Strontium is a metal, but as such has found little practical use. Most of its compounds are similar to those of calcium.
Strontium nitrate Sr(NO3)2 is mixed with carbon and sulfur to make red fire for use in fireworks, flares, and signal shells. Strontium chlorate, Sr(ClO3)2 can be used for the same purpose. One mineral form of strontium is a crystalline form of strontium sulfate called celestite. Others include the carbonate minerals strontianite and Weloganite. Strontium appears in the carbonate mineral Bensonite.
Strontium appears with boron in the oxide mineral tunellite and with barium in the silicate minerals Brewsterite and heulandite.
The radioisotope strontium-90 has been problematic in the fallout from nuclear weapons testing and nuclear accidents. Its intermediate halflife makes it quite radioactive, and since its compounds mimic those of calcium, it is taken up and reconcentrated in living organisms.
|