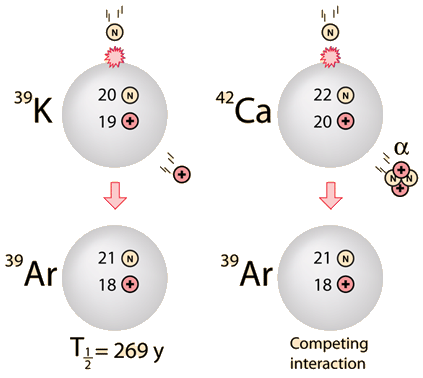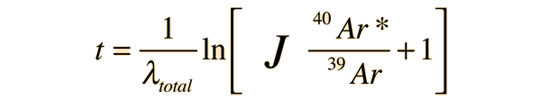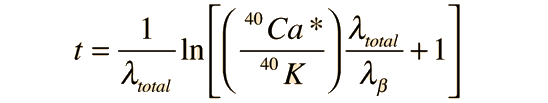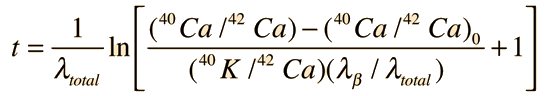Potassium-Argon Dating
Potassium-Argon dating has the advantage that the argon is an inert gas that does not react chemically and would not be expected to be included in the solidification of a rock, so any found inside a rock is very likely the result of radioactive decay of potassium. Since the argon will escape if the rock is melted, the dates obtained are to the last molten time for the rock. Since potassium is a constituent of many common minerals and occurs with a tiny fraction of radioactive potassium-40, it finds wide application in the dating of mineral deposits. The feldspars are the most abundant minerals on the Earth, and potassium is a constituent of orthoclase, one common form of feldspar.
 | 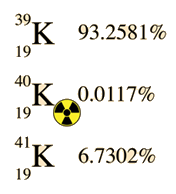 | Potassium occurs naturally as three isotopes. The radioactive potassium-40 decays by two modes, by beta decay to 40Ca and by electron capture to 40Ar. There is also a tiny fraction of the decay to 40Ar that occurs by positron emission. The calcium pathway is not often used for dating since there is such an abundance of calcium-40 in minerals, but there are some special cases where it is useful. The decay constant for the decay to 40Ar is 5.81 x 10-11yr-1. |
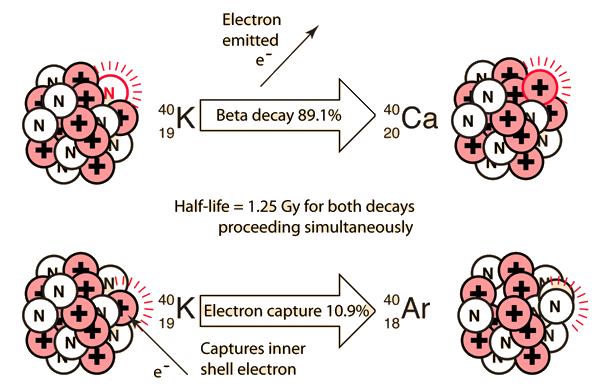
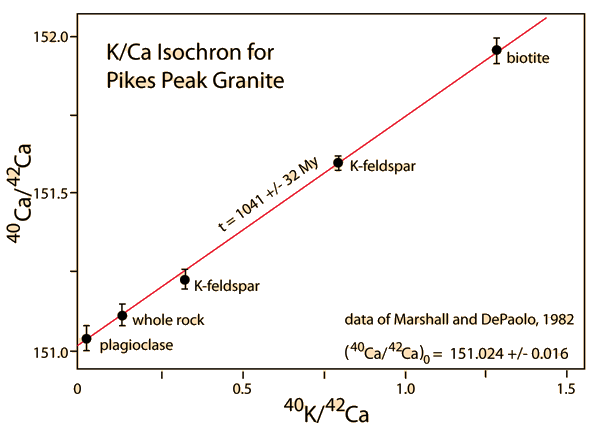
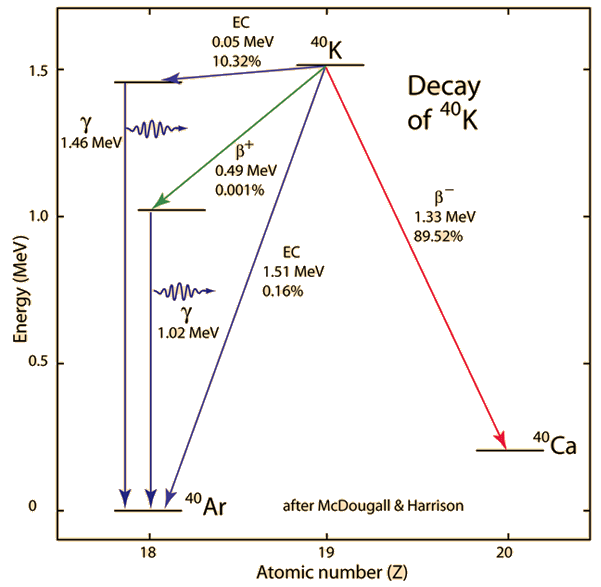
Even though the decay of 40K is somewhat complex with the decay to 40Ca and three pathways to 40Ar, Dalrymple and Lanphere point out that potassium-argon dating was being used to address significant geological problems by the mid 1950's. The energy-level diagram below is based on data accumulated by McDougall and Harrison.
For a radioactive decay which produces a single final product, the decay time can be calculated from the amounts of the parent and daughter product by
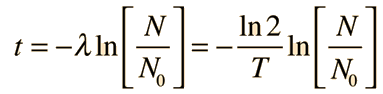
where N0 and N are the initial and final numbers of the parent isotope, λ is the decay constant and T is the half-life. But the decay of potassium-40 has multiple pathways, and detailed information about each of these pathways is necessary if potassiun-argon decay is to be used as a clock. This information is typically expressed in terms of the decay constants.
The measured amount of radiogenic 40Ar* in terms of the current measured amount of 40K can be expressed as
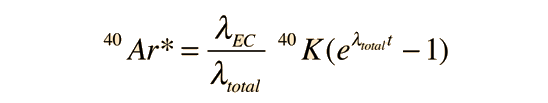
This can be solved for the time t
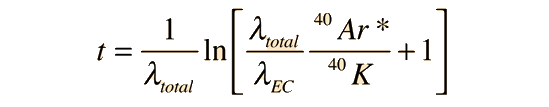
When the values for the decay constants in the table above are used, the expression for the radiometric age becomes
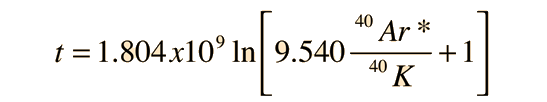
Here, it is useful to make use of the series representation of ln(x+1), which may be approximated by x if x<< 1:
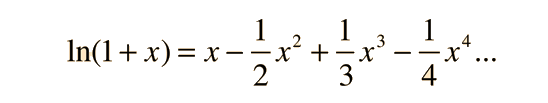
Since the population of 40Ar* is usually quite small, the approximation of ln(x+1)≈x gives
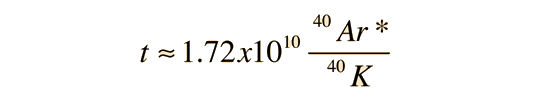
Geyh & Schleicher comment that the above approximation results in only 1% error at 107 years.
* The asterisk in 40Ar* is a reminder that a valid date is obtained only if all the argon-40 is of radiogenic origin in that particular sample. The assumptions made are
- When the radiometric clock was started, there was a negligible amount of 40Ar in the sample.
- The rock or mineral has been a closed system since the starting time.
- The closure of the system was rapid compared to the age being determined.
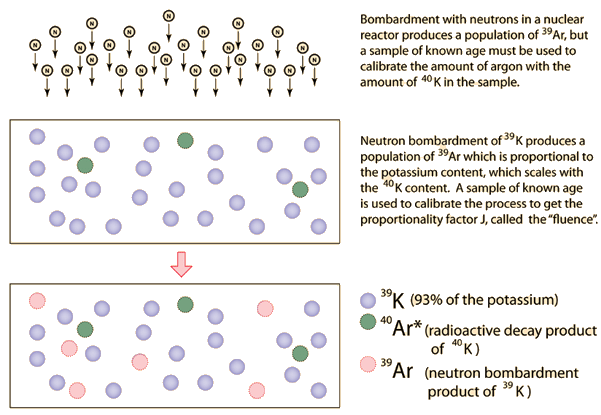
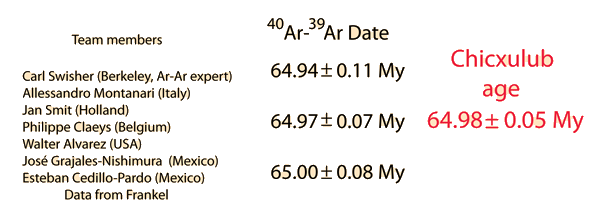
| 40Ar/39Ar Geochronology |
| Clocks in the Rocks |
McDougall & Harrison
Dalrymple & Lanphere
Geyh & Schleicher, Ch 6
| HyperPhysics***** Nuclear | R Nave |