Graphite
 | CThese samples of graphite are displayed in the Smithsonian Museum of Natural History. Graphite is a form of carbon. The sample at left is about 12 cm across and is from Canada. |
|
Carbon in the graphite form is made up of layers of carbon atoms which can be induced to move relative to each other. It is used as a dry lubricant. It is much softer than carbon in the diamond form, which is the hardest mineral. Graphite has a hardness of 1-2 compared to 10 for diamond. | 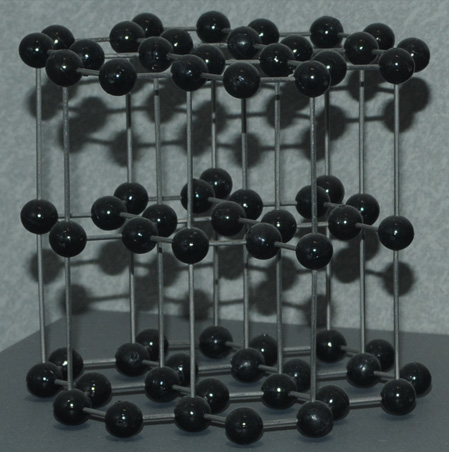 |
 |
This sample is graphite with calcite from Ticonderoga, New York. The sample is about 15 cm wide. |
Mindat: Graphite
| Minerals |
| HyperPhysics*****Geophysics | R Nave |