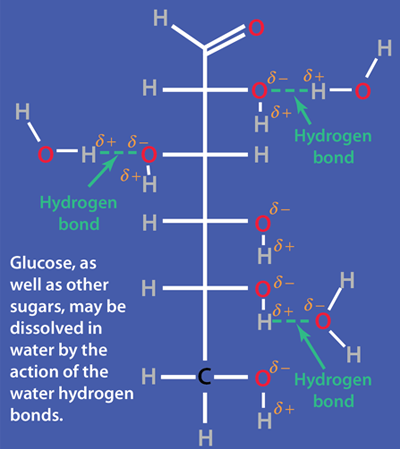
Water, the Solvent for Life
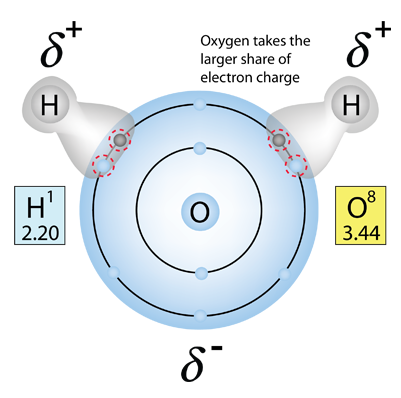
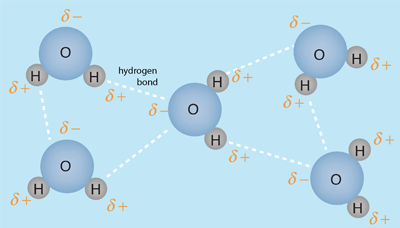
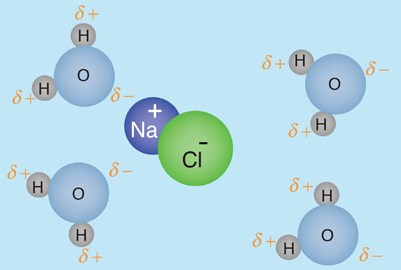
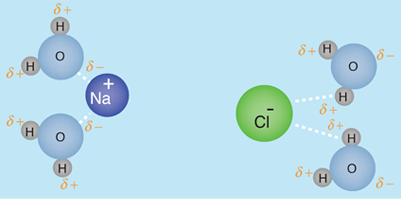
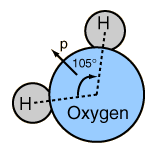
The human body is 66% water by weight, according to Hill and Kolb. Water is the universal solvent for life, referred to by Nobel Laureate A. Szent-Gyorgy as "the matrix of life".That water serves as the solvent for sodium chloride (salt) and other substances so that the fluids of our bodies are similar to sea water. This leads Hill and Kolb to refer jokingly to us as "walking bags of sea water". Water serves to suspend the red blood cells to carry oxygen to the cells. It is the solvent for the electrolytes and nutrients needed by the cells, and also the solvent to carry waste material away from the cells.
With water as the solvent, osmotic pressure acts to transport the needed water into cells. With cells bathed in the interstitial fluid, diffusion contributes to carrying needed molecules into the cells. When more complex mechanisms control the transport of molecules across the membranes into and out of cells, the presence of water as the surrounding medium and solvent is essential.
|
Water concepts
Chemistry concepts
Reference
Hill & Kolb
Ch 13 "Water"
| HyperPhysics*****Chemistry | R Nave |