Stark Effect in Atomic Spectra
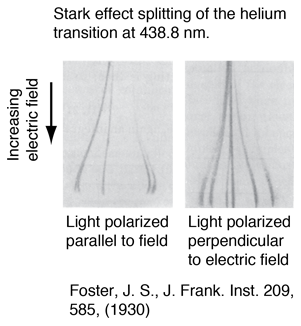 |
The splitting of atomic spectral lines as a result of an externally applied electric field was discovered by Stark, and is called the Stark effect. As the splitting of a line of the helium spectrum shows, the splitting is not symmetric like that of the Zeeman effect. The splitting of the energy levels by an electric field first requires that the field polarizes the atom and then interacts with the resulting electric dipole moment. That dipole moment depends upon the magnitude of Mj, but not its sign, so that the energy levels show splitting proportional to quantum numbers J+1 or J+1/2, for integer and half-integer spins respectively. The Stark effect has been of marginal benefit in the analysis of atomic spectra, but has been a major tool for molecular rotational spectra. |
Atomic Structure Concepts
Reference
Herzberg
Atomic Spectra
Ch 2, p 114.
| HyperPhysics***** Quantum Physics | R Nave |