Chemical Shifts in NMR Spectra
The signal frequency that is detected in nuclear magnetic resonance (NMR) spectroscopy is proportional to the magnetic field applied to the nucleus. This would be a precisely determined frequency if the only magnetic field acting on the nucleus was the externally applied field. But the response of the atomic electrons to that externally applied magnetic field is such that their motions produce a small magnetic field at the nucleus which usually acts in opposition to the externally applied field. This change in the effective field on the nuclear spin causes the NMR signal frequency to shift. The magnitude of the shift depends upon the type of nucleus and the details of the electron motion in the nearby atoms and molecules. It is called a "chemical shift". The precision of NMR spectroscopy allows this chemical shift to be measured, and the study of chemical shifts has produced a large store of information about the chemical bonds and the structure of molecules.
The effective magnetic field at the nucleus can be expressed in terms of the externally applied field B0 by the expression
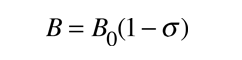
where σ is called the shielding factor or screening factor. The factor σ is small - typically
10-5 for protons and <10-3 for other nuclei (Becker).
In practice the chemical shift is usually indicated by a symbol δ which is defined in terms of a standard reference.
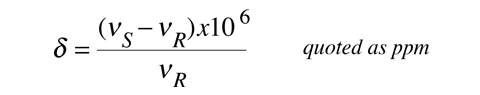
The signal shift is very small, parts per million, but the great precision with which frequencies can be measured permits the determination of chemical shift to three or more significant figures. The reference material is often tetramethylsilane, Si(CH3)4, abbreviated TMS. Since the signal frequency is related to the shielding by
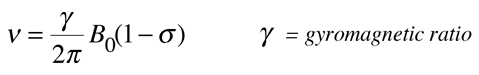
the chemical shift can also be expressed as
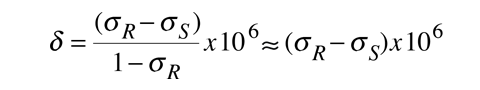
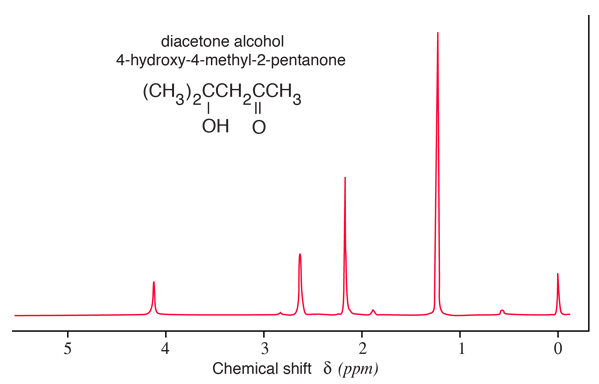
A sample of a chemical shift spectrum is given below. It is a sketch of a proton nmr spectrum included in Becker, Chapter 1. The high-resolution peaks can be identified with the functional groups in the molecule: δ = 1.23, (CH3)2; 2.16, CH3C=O; 2.62,CH2; 4.12, OH.
| Chemical applications of NMR |
Nuclear Spectra Concepts
References
Hobbie
Ch 17
Becker
Ch 1,4
| HyperPhysics***** Nuclear | R Nave |