Rubidium/Strontium Dating of Meteorites
The study of the rubidium/strontium isotopic ratios in a set of meteorite samples shows the general approach to this kind of radioactive dating. The isotope 87Rb decays into the ground state of 87Sr with a half-life of 4.7 x 1010 years and a maximum b- energy of 272 keV. 86Sr is of non-radiogenic origin and can be used as a reference concentration to imply the original concentration of 87Sr.
From an example by Jelley, the following five chondritic meteorites are reported to have the following proportions of the rubidium and strontium isotopes:
| Meteorites | ||
| Modoc | ||
| Homestead | ||
| Bruderheim | ||
| Kyushu | ||
| Buth Furnace |
This data was plotted with the spreadsheet Excel as shown below.
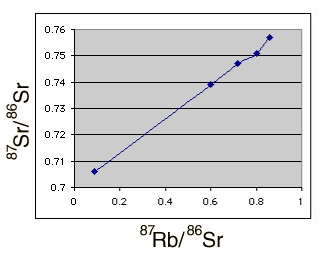
The age consistent with the slope of this plot can be calculated in a way which makes use of the non-radiogenic 86Sr as a reference. The slope obtained was m=0.0676, using the point-slope form of a line, y = mx + b, and the corresponding age is 4.6 x 109 years. The intercept b = 0.70 suggests that these values for isotopic abundances are consistent with an original strontium isotope ratio 87Sr/ 86Sr = 0.7 .
While the consistency is remarkable, there are uncertainties. A glance at the plot above shows that the Homestead meteorite is a bit off the line with the others. In fact, if just an average of the point-to-point slopes for all the data points is used, including the Homestead meteorite, an age of 4.7 billion is obtained, showing that there is a significant uncertainty in the dates obtained. The 4.6 billion year value was obtained by eliminating the Homestead meteorite, since that appeared to give a more consistent slope for the curve. This is all the data I have about these particular samples - comments about this scenario are invited.
Reference
Jelley
Sec 3.8
| HyperPhysics***** Nuclear | R Nave |