Isotopic Abundances by Mass Spectrometry
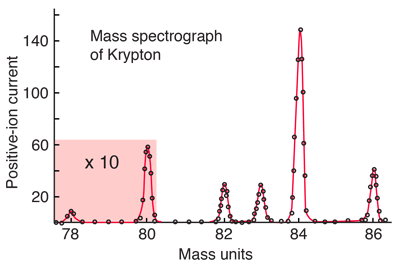
The relative abundances of the isotopes of an element may be obtained with a mass spectrometer. For example, the relative abundances of krypton are shown below on an experimental spectrum adapted from Krane, Introductory Nuclear Physics.
 |
|
A weighted average of the isotopes above gives 83.8 u, the accepted atomic mass of krypton which appears in the periodic table. Other isotopes of krypton are known, but they do not appear in natural samples because they are unstable (radioactive).
| Atoms and elements |
Reference
Krane
Ch 3
| HyperPhysics***** Nuclear | R Nave |