Electron Scattering from Nuclei
The scattering of electrons from nuclei has given us the most precise information about nuclear size and charge distribution. The electron is a better nuclear probe than the alpha particles of Rutherford scattering because it is a point particle and can penetrate the nucleus.
For low energies and under conditions where the electron does not penetrate the nucleus, the electron scattering can be described by the Rutherford formula. The Rutherford formula is an analytic expression for the differential scattering cross section, and for a projectile charge of 1, it is
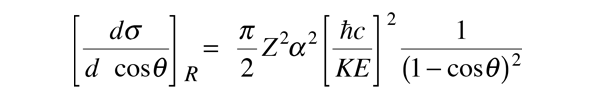
As the energy of the electrons is raised enough to make them an effective nuclear probe, a number of other effects become significant, and the scattering behavior diverges from the Rutherford formula. The probing electrons are relativistic, they produce significant nuclear recoil, and they interact via their magnetic moment as well as by their charge. When the magnetic moment and recoil are taken into account, the expression is called the Mott cross section:
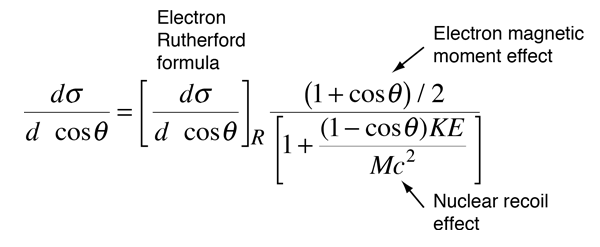
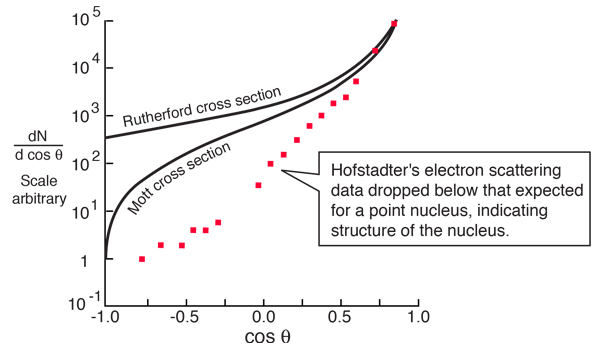
A major period of investigation of nuclear size and structure occurred in the 1950's with the work of Robert Hofstadter and others who compared their high energy electron scattering results with the Mott cross section. The illustration below from Hofstadter's work shows the divergence from the Mott cross section which indicates that the electrons are penetrating the nucleus - departure from point-particle scattering is evidence of the structure of the nucleus.
This productive period of research with electron scattering built the picture of the nucleus as a spherical distribution of positive charge of essentially constant density, so that the nuclear radius could be modeled by the relationship:
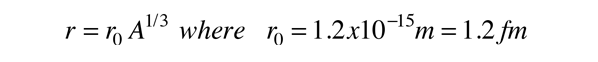 |
|
confirming the Fermi model for the nuclear radius.
Rutherford concepts
Reference
Rohlf
Ch 6.
| HyperPhysics***** Nuclear | R Nave |