The Mass-5 Roadblock
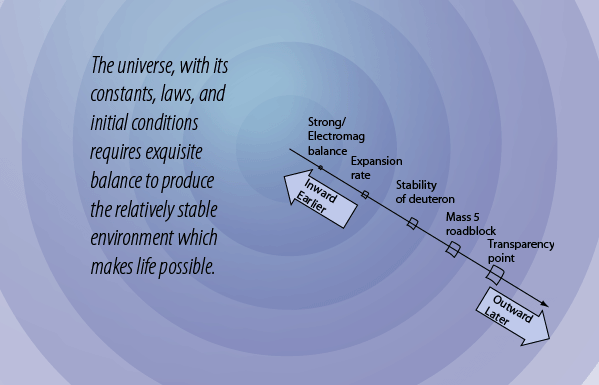
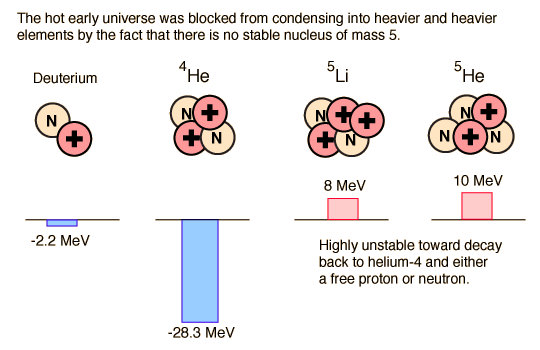
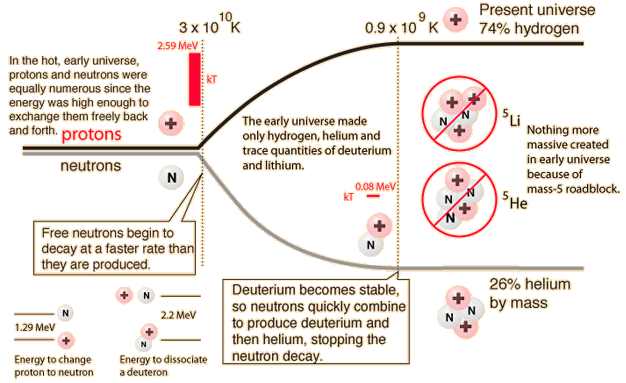
In order for us to exist, we had to have a star, the Sun, which is overwhelmingly composed of hydrogen and helium. But after the deuterons formed and subsequently formed helium nuclei (alpha particles), the "clumping" process to make more massive nuclei stopped. Why? Because there is no stable nucleus of mass 5. The energies involved are illustrated in the diagram above. The -2.2 MeV of energy associated with deuterium shows that it has a negative binding energy and is thus stable. But this is 2.2 MeV out of a total mass energy of about 1876 MeV for the deuteron, so it is a relatively thin margin of stability, and became stable only when the expanding universe cooled to near 109K. The deuterium in the early universe combined quickly to form helium which can be seen to be exceptionally stable. But the mass-5 nuclei are highly unstable, so no other nuclei formed except trace quantities of 7Li and 7Be. The formation of heavier elements had to await the operation of the nuclear fusion furnaces in the center of stars.
The deuteron is stable because in experiencing the strong nuclear force between the neutron and proton, there is a mass reduction associated with the nuclear binding energy which makes the deuteron's mass smaller than the sum of the neutron and proton mass. It is then "uphill" in energy by 2.2 MeV for it to separate back into a proton and neutron.
Once the deuterons are formed, they have a very strong tendency to combine into helium nuclei . The helium nucleus (alpha particle) is very tightly bound and the rapid process of combining deuterons into helium nuclei gives us the cosmic abundance of hydrogen and helium we have today. But with the helium nuclei a new factor comes into play - the balance between the repulsive electromagnetic force and the attractive nuclear strong force. This intense conflict between the two strongest forces determines the stability of all nuclei more massive than deuterium. In the case of the helium nucleus, the strong force is dominant and it forms a very stable nucleus.
But the next step brings a surprise. Adding a neutron to make helium-5 does not yield a stable nucleus. Adding a proton makes lithium-5 which is likewise unstable. Adding a deuteron to make lithium-6 might work, but it is less stable than the helium nucleus and its probability is extremely low. So the process of heavy element building is effectively roadblocked at some three minutes into the age of the universe! It is not until the universe has expanded and cooled sufficiently to allow atoms to form, and those atoms in sufficient concentrations to colllapse to form stars and kindle the fires of nuclear fusion, is the progression of heavy element building resumed.
Consider the scenario where the strong nuclear force was relatively stronger compared to the electromagnetic force. Then the clumping into ever more massive nuclei would have continued and the universe would have been made of heavy elements instead of hydrogen and helium. Then there would have been no stars, and no "us".
| The decoupling of radiation in the early universe. |
HyperPhysics  | R Nave |